In addition to already being recognized as a Notified Body for the European EMC & RED Directives, LabTest Certification is now also recognized as an Approved Body (AB 2815) for the UK. This official status under the UK’s Radio Equipment Regulations 2017 (SI 2017/1206) and Electromagnetic Compatibility (EMC) Regulations 2016 (SI 2016/1091) allows us to provide the necessary conformity assessment for the U.K. market.
Understanding UKCA Marking: LabTest Certification Now an Approved Body for the UK Market
Following the United Kingdom’s departure from the European Union, a new regulatory framework for products has been established. The UKCA (UK Conformity Assessed) marking is the new product marking used for goods being placed on the market in Great Britain (England, Wales, and Scotland). It replaces the requirement for the CE marking for most products covered by previous EU directives.
What does that mean?
We can assist manufacturers with product approvals for the UKCA mark and also help with updating current CE Marking technical files to the new UKCA files.
Worried about the new UKCA process?
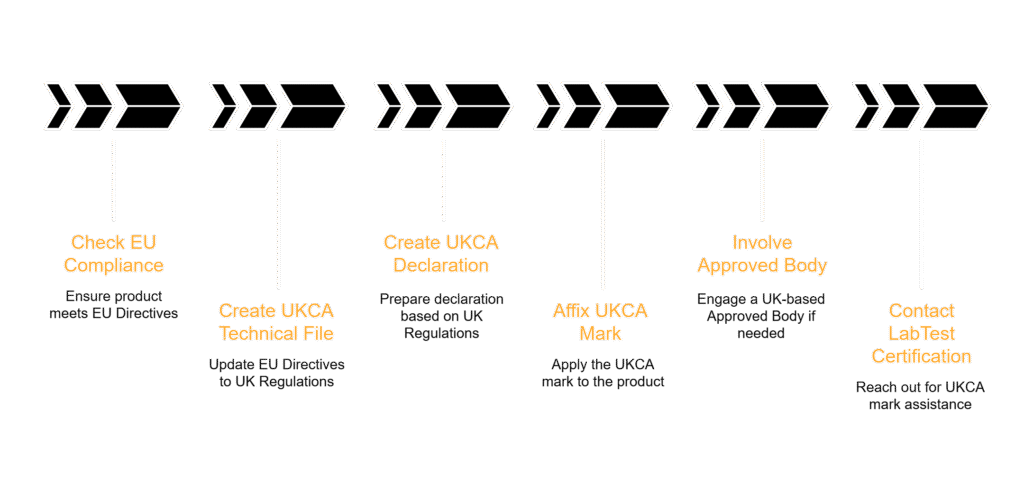
For manufacturers, the process of conformity assessment for the U.K. is often straightforward, especially if a product is already compliant with the corresponding EU directives. If products already comply with the EU Directives and you have used the EN harmonized standards before… The process is simple!
- Create new UKCA Technical File based on your CE marking Technical File:
- Update the EU Directives to the UK Regulations
- Update the EN standards to BS Standards
- Create new UKCA Declaration, based on the UK Regulations and BS Standards
- Affix UKCA Mark on the product
- Mandatory from January 1, 2022
In situations where the EU Directive(s) required the involvement of a Notified Body from within the European Union, as registered in the NANDO database, the U.K. Regulations will also require an Approved Body located within the U.K. to be involved for similar situations.
Are you ready to sell to the U.K. market? Contact LabTest Certification at info@labtestcert.com today to get the UKCA mark on your products.
Mark these dates!
Adherence to the following timelines is critical for maintaining market access in the UK:
January 1, 2021
- 12-month transition period starts
- CE Marking is still accepted in the UK
- UKCA marking is being accepted in the UK
January 1, 2022
- CE Marking and CE Declaration not accepted in the UK (Very few exceptions permitted)
- UKCA Declaration mandatory
January 1, 2023
- Products shall be affixed with the UKCA Mark
- UKCA Declaration mandatory
Next Steps for Manufacturers
With these deadlines now in effect, ensuring compliance is a immediate priority for sales into the UK market. LabTest Certification’s dual accreditation as both an EU Notified Body and a UK Approved Body offers a significant advantage, providing a single source for guidance on both regulatory landscapes.
Upcoming deadlines are approaching fast!